From November 17-20, 2025, the world-renowned MEDICA Exhibition concluded triumphantly in Düsseldorf, Germany, solidifying its reputation as the global benchmark for medical B2B trade events. Following meticulous planning and innovation-driven preparation, the Hipro team delivered a standout performance at this year’s edition, showcasing cutting-edge in vitro diagnostic (IVD) solutions that underscored the company’s technical prowess and commitment to advancing healthcare accessibility.
As the largest medical technology exhibition worldwide, MEDICA 2025 welcomed over 5,300 exhibitors from 76 countries and drew more than 85,000 professional visitors—setting new records for participation. The four-day event served as a dynamic hub for industry leaders, featuring exhibitions, high-level forums, and technical workshops focused on key trends including digital transformation, precision medicine, and sustainable medical technologies. Covering medical devices, diagnostics, digital health, and hospital infrastructure, MEDICA 2025 continued to drive global collaboration and innovation in the healthcare sector.
As a front-runner in the global IVD industry, Hipro’s booth became a focal point of the exhibition, attracting constant foot traffic from healthcare professionals, distributors, and industry partners. The team’s in-depth technical demonstrations and interactive product experiences sparked enthusiastic discussions, reinforcing Hipro’s position as an innovative force pushing the boundaries of diagnostic technology. This year’s participation further strengthened the company’s international network and paved the way for strategic partnerships across EMEA, Asia-Pacific, and the Americas.
.jpg)
Specific Protein Detection Series: A1 & A1 Plus
Hipro’s liquid-based specific protein testing solutions—flagship models A1 and A1 Plus—remained a highlight of the booth. Renowned for excellence in specific protein detection, these products are tailored to meet the varying demands of medical institutions and laboratories, with each model boasting unique features designed for different operational needs. The A1, in particular, continued to draw considerable attention from professionals and buyers, building on its established popularity across European, Middle Eastern, Southeast Asian, and South Asian markets since its launch. Both models uphold Hipro’s commitment to delivering consistent, high-performance diagnostic tools for clinical settings
.jpg)
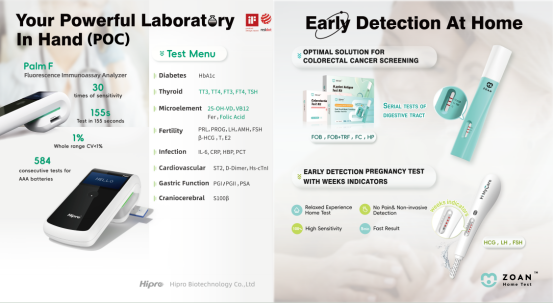
Immunofluorescence: European Market Sensation
The PalmF portable diagnostic device—designed for primary care organizations and personal health management—reinforced its strong foothold in the European market. This compact, portable device supports tests for diabetes, cardiac conditions, vitamins, and female reproductive hormones, facilitating accessible health diagnostics for all. Enjoying immense popularity among European buyers, all demonstration units brought to MEDICA 2025 were fully purchased by interested clients, underscoring its unrivaled market appeal. Building on successful collaborations in Germany, Italy, Switzerland, and the Netherlands—including existing OEM agreements—Hipro continues to strengthen its presence across the European market.
.jpg)
Microfluidics System: Rapid, Reliable Emergency Diagnostics
Hipro’s advanced microfluidics system—another core innovation in its IVD portfolio—remained a key attraction. By precisely controlling the flow of small liquid volumes within microchannels, this technology enables fast and efficient detection of biological samples, improving test sensitivity and specificity while significantly reducing testing time. Critical for emergency and critical care settings where quick, accurate results are vital, the system has maintained its reputation as a game-changer in urgent diagnostic scenarios. Its proven performance continues to resonate with healthcare providers seeking reliable, time-saving solutions.

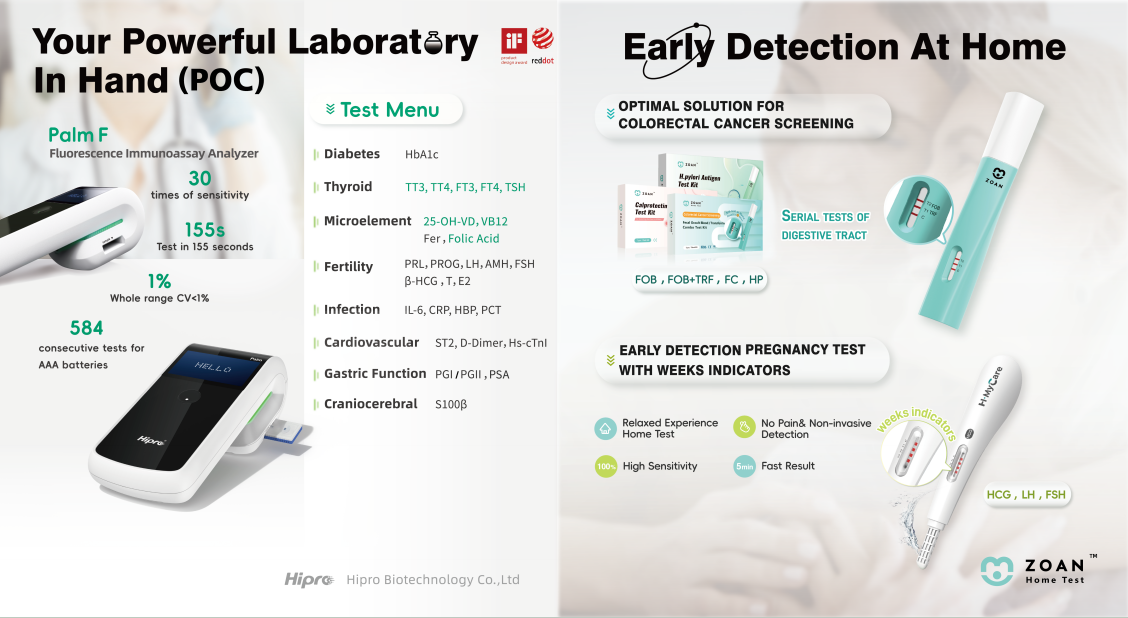
ZOAN Home Test Series: Trusted Gastrointestinal Health Monitoring
Hipro’s ZOAN home test series—focused on digestive tract health monitoring—garnered widespread acclaim at the exhibition. Praised for its ergonomic design and intuitive operation, the series enables customers to monitor their gastrointestinal health comprehensively and timely from the comfort of home. The product’s user-friendly nature and clinical-grade accuracy resonated strongly with visitors: all samples were fully claimed by interested clients during the event, a testament to market recognition of its practicality and reliability. By offering accessible at-home solutions, Hipro empowers individuals to take proactive steps in managing their digestive health with ease and precision.
Hipro’s participation in MEDICA 2025 reaffirms our unwavering commitment to delivering trusted IVD solutions and addressing global healthcare needs. The enthusiastic response to our product portfolio—including the sell-out of PalmF samples and strong demand for ZOAN—underscores that our focus on precision, accessibility, and user-centric design resonates deeply with industry partners worldwide.
We extend sincere gratitude to all visitors, distributors, and experts who engaged with our team. Your insights fuel our dedication to refining our offerings and expanding our global footprint. Building on this momentum, Hipro will continue advancing reliable diagnostic solutions that enhance patient care and streamline clinical workflows.
We warmly invite you to join us at MEDICA 2026 in Düsseldorf to explore deeper collaborations and celebrate ongoing excellence in in vitro diagnostics. See you next year!
