Warm congratulations to Hipro that the 2019-nCoV IgM /IgG Antibody Test Kit (Colloidal Gold ) for obtaining the registration certificate from the Brazilian Health Regulatory Agency (ANVISA)!
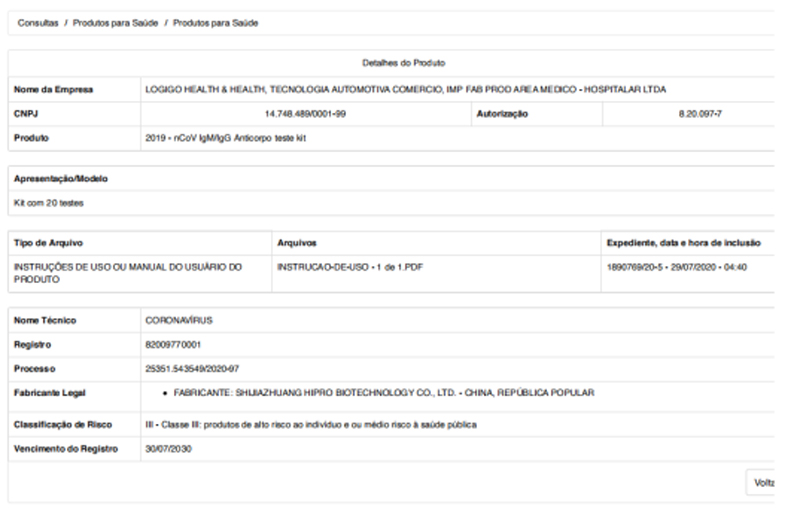
ANVISA, whose full name is Agência Nacional de Vigilância Sanitária, is affiliated with the Brazilian Ministry of Health and is mainly responsible for pre and post-marketing supervision of medical supplies including in vitro diagnostic products. It is equivalent to the Food and Drug Administration (FDA) of the United States and the National Medical Products Administration (NMPA) of China. All import and distribution of medical items in Brazil must be certified by ANVISA.


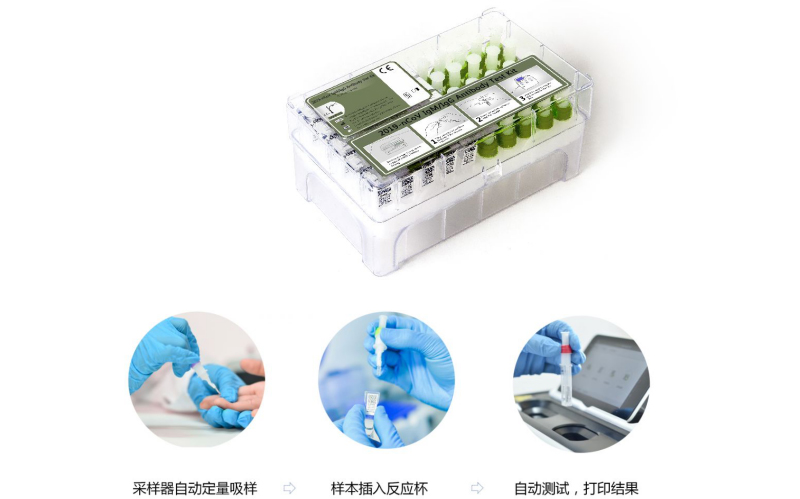
Operation diagram:
Specimen: Whole blood/serum/plasma
Methodology: Colloidal Gold
Test time: 10mins
Operation diagram:
Specimen: Serum/plasma
Methodology: Nephelometry Immunoassay Method
Test time: 6mins



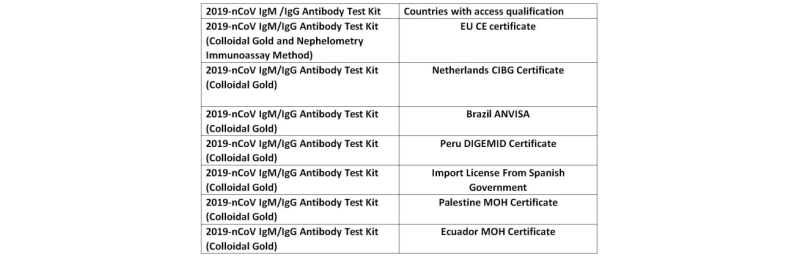
Currently, the 2019-nCoV IgM/IgG Antibody Test Kit from Hipro has been certified (authorized) by medical regulatory agencies in many countries and regions such as the European Union, the Netherlands, and other countries and regions, and have been sold in the above regions. This time, obtaining the registration certificate from the Brazilian Health Regulatory Agency (ANVISA), further expanded the scope of the sales and made greater contributions to global epidemic prevention and control.